By Chris Evers, Contributing Author
As the global concern over plastic pollution intensifies, attention has shifted from the visible litter we think of to microplastics.
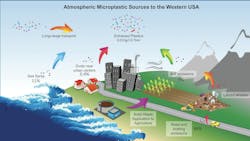
While the focus often centers on their effects on marine ecosystems, a significant issue has emerged closer to home for the heavy highway industry: the majority of microplastics originate from our surface infrastructure, primarily through tire and brake wear.
The long-term practice of incorporating polymers, crumb rubber, and more recently recycled polyethylene (PET) or polypropylene (PP) into pavement materials to enhance the durability and longevity of roads further complicates the issue.
This poses multifaceted challenges to the integrity and sustainability of our road associated microenvironment. Within the realm of road-related environmental concerns, a relatively recent addition is the issue of microplastic contaminations.
Researchers now draw parallels between the widespread utilization of plastics and the establishment of a global "plastic cycle," comparable in significance to other human-influenced processes like the carbon cycle.
The proliferation of microplastic particles (MPP) poses alarming health and ecological risks, as individuals inadvertently ingest these particles through breathing, eating, and drinking.
Consequently, the escalating levels of MPP present growing concerns regarding their potential effect. Much attention has been paid to the “Great Pacific Garbage Patch” that covers an area twice the size of Texas. However, MPP poses a more subtle and insidious risk due to its widespread distribution, miniscule size, and ever-present source.
A 2020 Cornell University study found that up to 85% of the microplastics found in our environment originate from roads, significantly contributing to poor air quality and water contamination. Cars and trucks are the primary culprits, with tire and brake-pad wear being significant contributors.
Scientists have coined a term for these MPP accumulations found on roadways: "road-associated microplastic particles" or RAMPs.
Researchers at U.K.-based Emissions Analytics found that non-exhaust emissions (NEE’s) could be as much as 1,000 times to 2,000 times worse than car exhaust.
In the coming years, wider adoption of electric vehicles, which are typically heavier than internal combustion engine vehicles, will increase tire and brake wear that in turn creates more RAMPs.
Michael Durante, vice president of strategy at Pavement Technology Inc., has been sounding the alarm at industry gatherings the last several years. He points out that one of the most overlooked concerns is that tire-wear debris is comprised of so-called “forever chemicals.”
These highly toxic fluorinated chemicals are categorized in the family of perfluoroalkyl and polyfluoroalkyl substances, and they are referred to as “PFAS.”
They end up in our air and water, thereby building up in wildlife and human organs. They never naturally break down in the environment.
PFAS is linked to reproductive problems, auto-immune deficiency diseases, and various forms of cancer. No product containing PFAS or a PFAS precursor releases more of this in the environment than on-road tire wear, according to the Environmental Protection Agency (EPA).
In tires, a processed chemical known as 6PPD is universally used as a preservative film or buffer against oxidation. It is effective at improving tire life as it aggressively interacts with ground-level ozone, thus protecting the tires.
However, when this film additive itself oxidizes it transforms into the 6PPD-Q, which is similar to a polycyclic aromatic hydrocarbon (PAH).**
These “forever chemicals” are lethal to various aquatic species at concentrations as low as 0.5 parts per billion and dangerous to humans over the long term.”
This is where the proverbial rubber meets the literal road.
Crumb rubber began appearing in the asphalt industry in the 1970’s and 1980’s to remove waste tires accumulating in landfills. Specifying the use of ground tire rubber (GTR) was even mandated by some departments of transportation (DOTs), a few of which persist today.
DOTs in California, Arizona, Texas, and Florida continue to encourage the use of GTR in varying degrees. Now with attention paid to the dangers of RAMPs and specifically PFAS in the environment, our industry faces competing environmental concerns.
Do we as an industry continue practices that increase the risk of greater harm to the environment and human health, or do we alter construction practices to mitigate that risk?
This issue becomes even more critical as newer methods of incorporating plastic waste come to market, particularly recycled post-consumer/post industrial waste materials such as PET and PP. Even the standard polymer additives like SBS and SBR polymers long used to modify asphalt binder aren’t escaping increased scrutiny.
Should distinctions be made on which of these asphalt binder modifications are allowed for surface courses and thereby exposed to traffic wear?
This question will take on greater significance in the coming years as DOT engineers and public works professionals grapple with the attention being paid to acronyms that even a few years ago were relatively unknown.
With stricter regulations on the horizon, particularly in communities near bodies of water, stormwater managers will be looking for answers for PFAS contamination emanating from roadways.
Dianne Kopec, a researcher and faculty fellow at the University of Maine who studies PFAS and mercury in wildlife, has warned that eating fish with high concentrations of PFAS may be more harmful than mercury.
What then is the solution? It’s nearly impossible to imagine meaningful reductions in RAMPs due to the omnipresent car and truck in modern society. “Forever chemicals” also seem to be a challenge to reduce significantly without a high economic cost in terms of lowered life cycle of tires.
Even EV adoption, which promises zero emissions, faces heightened examination caused by greater tire wear thanks to their increased gross weight. And while cleaning up the Great Pacific Garbage Patch is a worthy effort, it’s only effective for larger plastic debris in our oceans — not MPPs that are found everywhere.
With a challenge of this magnitude, a single solution does not exist.
The heavy highway industry and stakeholders only share a limited amount of the burden since much of the issue resides with tire and brake manufacturers, industry, consumers, and government.
However, there are strategies we can deploy as specifying agencies and contractors that go a long way to mitigate such a massive and underestimated problem. With overwhelming research illustrating the magnitude of the issue and media reporting on the widespread evidence of microplastics permeating the food supply, consensus on the importance of acting is present.
The lowest hanging fruit appears to be materials selection and roadway design. If government agencies insist on encouraging the use of recycled rubber and plastics, it is easier to mitigate second and third order negative effects by shifting pavements with these materials into the base course segment of design.
Using GTR or PET/PP recycled modifiers in asphalt binder could be safer in between aggregate base and the asphalt surface course for thicker pavements. Avoiding the use of surface courses that have the potential to wear under traffic and generate RAMPs from GTR or PET/PP would be a straightforward strategy.
Technology holds tremendous promise, and here is where high school chemistry reappears after a long hiatus.
The less famous brother of photosynthesis has begun to emerge across several platforms as a potential solution to a broad spectrum of challenges. It’s called photocatalysis or photocatalytic oxidation (PCO).
It occurs when a catalyst, most commonly photoreactive titanium dioxide (TiO2) that’s been added to a road, building, roof, glass, etc., becomes solar energized.
When TiO2 is exposed to light, it releases electrons that combine with oxygen to create superoxide anion (HO2-). The catalyst then attracts electrons from moisture in the air to replenish its own supply.
As a result, the TiO2 returns to its original state without being consumed. The moist air without electrons turns into hydroxyl radicals (-OH). These oxyradicals, including HO2- and -OH, gather on and around the treated surface like TiO2 generated piranhas that aggressively attack the carbon-hydrogen bonds present in all organic materials, producing complete oxidative decomposition of nearfield pollutants.
That includes RAMPs. A recent Texas A&M Transportation Institute study shows MPP decomposition efficiency of 99% with TiO2.
The co-benefits of utilizing PCO in roadway design is that photocatalysis targets GHG emissions such as CO2, criteria air pollutants like NOx while decomposing harmful pollutants such as RAMPs and PFAS.
It also helps to mitigate the urban heat island effect by reflecting much of the UV radiation that heats our pavements causing warmer urban environments.
This natural, air-purifying accelerant can retrofit existing pavements in a cost-effective way through spray applied applications which deliver the TiO2 to the surface of any pavements (or when properly incorporated in other construction materials).
As the heavy highway industry has done in the past, we need not look any further than intelligent roadway design and some high school chemistry to find a way to ride to the rescue rather than take blame for our part of the RAMP/PFAS issue.
Private and public stakeholders have already begun the discussions about shifts in pavement design and are beginning to deploy PCO treatments to tackle these most vexing challenges. Now the hard work of enabling the solutions of tomorrow to bear fruit today begins. RB
Chris Evers is the Technical Representative for PTI based out of Vero Beach, FL. He has 28 years of experience in the Heavy Highway industry and is currently the Executive Coordinator for the Florida Pavement Preservation Council. In 2023, Chris was honored with the APWA Top Ten Public Works Leader of the Year Award.
** In an eariler version of this story, as well as in the printed issue of the magazine, PFAS was incorrectly written in front of "6PPD-Q" in this sentence.